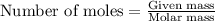Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:50, kyleighmarie05
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3


Chemistry, 22.06.2019 08:00, mariamakonteh31
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1
You know the right answer?
Write the balanced equation for the reaction of aqueous Pb ( ClO 3 ) 2 with aqueous NaI . Include ph...
Questions in other subjects:

Mathematics, 21.10.2021 04:50

Mathematics, 21.10.2021 04:50



Mathematics, 21.10.2021 04:50


History, 21.10.2021 04:50



Mathematics, 21.10.2021 05:00

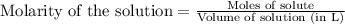
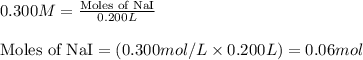
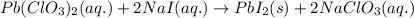
 of lead (II) iodide
of lead (II) iodide