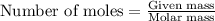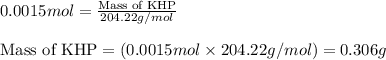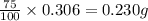Chemistry, 25.02.2020 20:53 lucywood2024
After doing multiple titrations, your NaOH solution is determined to have a mean concentration value of 0.100 M. Given you are to assume your unknown acid is 75.0% KHP, how many grams of your unknown will you need to use 15.00 mL of your 0.100 M standardized NaOH

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, cbelew0001ouje4i
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1


Chemistry, 22.06.2019 15:50, Edwardwall
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 20:30, dinapaul424
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1
You know the right answer?
After doing multiple titrations, your NaOH solution is determined to have a mean concentration value...
Questions in other subjects:


Chemistry, 02.09.2019 03:30

History, 02.09.2019 03:30


Mathematics, 02.09.2019 03:30



Mathematics, 02.09.2019 03:30


Mathematics, 02.09.2019 03:30

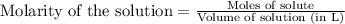
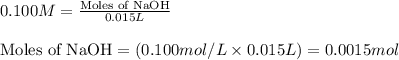
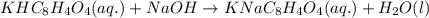
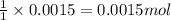 of KHP
of KHP