Consider a transition of the electron in the hydrogen atom from n=3 to n=7.
a) Determine...


Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, esnyderquintero
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 13:30, citlalli30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 23.06.2019 04:00, izzyp619
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3

Chemistry, 23.06.2019 07:00, jaydenboi604
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample?
Answers: 1
You know the right answer?
Questions in other subjects:



History, 02.07.2019 09:00



Social Studies, 02.07.2019 09:00

Chemistry, 02.07.2019 09:00

Computers and Technology, 02.07.2019 09:00




 = Wavelength of radiation
= Wavelength of radiation = Rydberg's Constant =
= Rydberg's Constant = 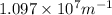
 = Higher energy level = 7
= Higher energy level = 7 = Lower energy level = 3
= Lower energy level = 3



