Chemistry, 25.02.2020 19:47 kaylailkanic1487
Be sure to answer all parts. For the titration of 10.0 mL of 0.250 M acetic acid with 0.200 M sodium hydroxide, determine the pH when: (a) 10.0 mL of base has been added. (b) 12.5 mL of base has been added. (c) 15.0 mL of base has been added.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, speris1443
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1


Chemistry, 22.06.2019 23:30, Xavier8247
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3

Chemistry, 22.06.2019 23:40, tilievaughn14
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1
You know the right answer?
Be sure to answer all parts. For the titration of 10.0 mL of 0.250 M acetic acid with 0.200 M sodium...
Questions in other subjects:


Computers and Technology, 01.08.2019 00:30

Chemistry, 01.08.2019 00:30

Mathematics, 01.08.2019 00:30

Mathematics, 01.08.2019 00:30





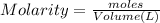
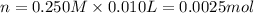
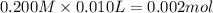
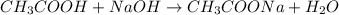
![[H^+]=\frac{0.0005 mol}{0.020 L}=0.025 M](/tpl/images/0523/5054/d4db0.png)
![pH=-\log[H^+]=-\log[0.025 M]=1.60](/tpl/images/0523/5054/f1f2d.png)
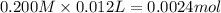
![[H^+]=\frac{0.0001 mol}{0.022 L}=0.0045 M](/tpl/images/0523/5054/5492d.png)
![pH=-\log[H^+]=-\log[0.0045 M]=2.34](/tpl/images/0523/5054/d5f92.png)
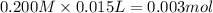
![[OH^-]=\frac{0.0005 mol}{0.025 L}=0.02 M](/tpl/images/0523/5054/d1bd6.png)
![pOH=-\log[OH^-]=-\log[0.02 M]=1.70](/tpl/images/0523/5054/8bd06.png)