
The reaction below is carried out at a different temperature at which Kc=0.055. This time, however, the reaction mixture starts with only the product, [NO]=0.0100M, and no reactants. Find the equilibrium concentrations of N2, O2, and NO at equilibrium. N2(g)+O2(g)⇌2NO(g)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, thechocolatblanc
During which movies do spring tides new moon first quarter waxing gibbous waxing
Answers: 1

Chemistry, 21.06.2019 18:10, hunterthompson2
Which is true of transition metals when moving from left to right on the periodic table? the d sublevels are not filled across the period. the cation radii become larger across the period. atomic radii increase slightly and then start to decrease. atomic radii decrease slightly and then start to increase. o
Answers: 2

Chemistry, 21.06.2019 18:20, datboyjulio21
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2

Chemistry, 22.06.2019 08:00, danielhall
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1
You know the right answer?
The reaction below is carried out at a different temperature at which Kc=0.055. This time, however,...
Questions in other subjects:










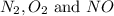 at equilibrium is, 0.0045 M, 0.0045 M and 0.001 M respectively.
at equilibrium is, 0.0045 M, 0.0045 M and 0.001 M respectively.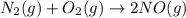
![K=\frac{[NO]^2}{[N_2][O_2]}](/tpl/images/0523/5256/ef70d.png)
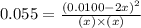
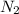 at equilibrium = x = 0.0045 M
at equilibrium = x = 0.0045 M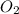 at equilibrium = x = 0.0045 M
at equilibrium = x = 0.0045 M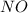 at equilibrium = (0.0100-2x) = (0.0100-2(0.0045)) = 0.001 M
at equilibrium = (0.0100-2x) = (0.0100-2(0.0045)) = 0.001 M

