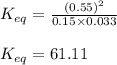Chemistry, 25.02.2020 19:21 vazquezemmy8
Consider the following chemical reaction: H2 (g) + I2 (g) 2HI (g) At equilibrium in a particular experiment, the concentrations of H2, I2, and HI were and respectively. The value of Keq for this reaction is.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, robert7248
What is the cellular process that releases the energy stored in food molecules
Answers: 3


Chemistry, 22.06.2019 03:00, cheesecake1919
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

You know the right answer?
Consider the following chemical reaction: H2 (g) + I2 (g) 2HI (g) At equilibrium in a particular exp...
Questions in other subjects:






English, 04.02.2020 12:57

Mathematics, 04.02.2020 12:57

Mathematics, 04.02.2020 12:57

History, 04.02.2020 12:58

 for the given reaction is 61.11
for the given reaction is 61.11 

![K_{eq}=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0523/4527/9c8b0.png)

![K_{eq}=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0523/4527/ad3b5.png)
![[HI]_{eq}=0.55M](/tpl/images/0523/4527/ad244.png)
![[H_2]_{eq}=0.15M](/tpl/images/0523/4527/4357e.png)
![[I_2]_{eq}=0.033M](/tpl/images/0523/4527/b80ab.png)