
A compound containing chromium, Cr; chlorine, Cl; and oxygen, O, is analyzed and found to be 33.6% chromium, 45.8% chlorine, and 20.6% oxygen by mass. What is the empirical formula of the compound? The molar mass of chromium, Cr, is 51.996 gmol; the molar mass of chlorine, Cl, is 35.45 gmol; and the molar mass of oxygen, O, is 15.999 gmol.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, vivianni0727p1y30v
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 07:30, tntaylor862
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 12:00, shifaxoxoxo
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2
You know the right answer?
A compound containing chromium, Cr; chlorine, Cl; and oxygen, O, is analyzed and found to be 33.6% c...
Questions in other subjects:


English, 02.09.2019 04:30

Geography, 02.09.2019 04:30



Mathematics, 02.09.2019 04:30

Computers and Technology, 02.09.2019 04:30

History, 02.09.2019 04:30


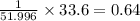 mol
mol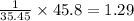 mol.
mol.
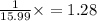 mol.
mol.
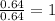
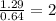
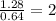
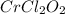
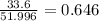
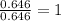 1
1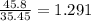
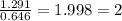 2
2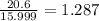
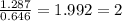 2
2

