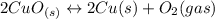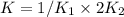Chemistry, 25.02.2020 06:14 andrewgainey1986
Given the equilibrium constants for the following reactions: 4Cu(s) + O2(g) <--> 2Cu2O(s), K1 2CuO(s) <--> Cu2O(s) + 1/2 O2(g), K2 what is K for the system 2Cu(s) + O2(g) <--> 2CuO(s) equivalent to?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, lasagnafoe
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 03:00, Dkhaurithompson
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1


Chemistry, 22.06.2019 15:30, alaf05160
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks. energy was destroyed inside the blocks. energy was absorbed into the blocks from outside the system. energy was transferred from the warmer block to the cooler block.
Answers: 2
You know the right answer?
Given the equilibrium constants for the following reactions: 4Cu(s) + O2(g) <--> 2Cu2O(s), K1...
Questions in other subjects:




Mathematics, 15.10.2019 08:50

Mathematics, 15.10.2019 08:50

Mathematics, 15.10.2019 08:50



History, 15.10.2019 08:50

Arts, 15.10.2019 08:50

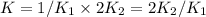
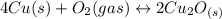
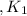 assuming equation (1)
assuming equation (1)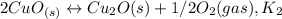 assuming equation (2)
assuming equation (2)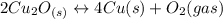 ,
,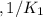 assuming equation (3)
assuming equation (3)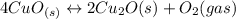
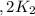 assuming equation (4)
assuming equation (4)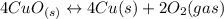
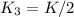 , equation (5)
, equation (5)