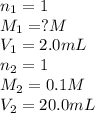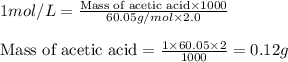Chemistry, 25.02.2020 05:12 rosetoheart2
You have a 2.0 mL sample of acetic acid (molar mass 60.05 g/mol) of unknown concentration. You titrate it to its endpoint with 20.0 mL NaOH (0.1 M). What mass of acetic acid was dissolved in the 2.0 mL of solution used?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, demetriascott20
How are ionic bonds formed and what is the attractive force within an ionic bond
Answers: 1


Chemistry, 22.06.2019 00:10, bossboybaker
Select the correct answer. which phrase correctly describes temperature? o a. average rotational kinetic energy of the particles in an object o b. average energy of the particles in an object c. average translational kinetic energy of the particles in an object od. all energy possessed by the particles in an object
Answers: 1

Chemistry, 22.06.2019 07:00, mayamabjishovrvq9
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1
You know the right answer?
You have a 2.0 mL sample of acetic acid (molar mass 60.05 g/mol) of unknown concentration. You titra...
Questions in other subjects:

History, 22.06.2019 11:00









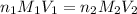
 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 
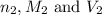 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.