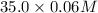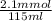Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, nayiiii1874
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 16:00, julesperez22
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 23.06.2019 00:50, kaseywright3418
Which statement would indicate the presence of an acid
Answers: 3

Chemistry, 23.06.2019 02:00, jacckiie5176
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2
You know the right answer?
If 35.0 mL of a 0.0500 M HNO3, 35.0 mL of a 0.0600 M KSCN, and 45.0 mL of a 0.0800 M Fe(NO3)3 are co...
Questions in other subjects:

Mathematics, 22.07.2019 17:20


Geography, 22.07.2019 17:20


Mathematics, 22.07.2019 17:20

Mathematics, 22.07.2019 17:20


Mathematics, 22.07.2019 17:20


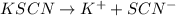
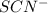 as follows.
as follows.