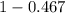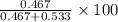A student was trying to determine the mole percent of A in a mixture of A and B using refractive index. If their mixture has a refractive index of 1.5248 and pure A and pure B each had refractive indices of 1.7058 and 1.3658, respectively, what was the mole percent of A in their mixture. Type your numerical answer rounded to the 3rd decimal place (i. e. 45.982 or 9.550, etc) without a percent sign.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:50, AysiaRamosLee
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 06:20, stephliu721
What is a property of a double replacement reaction
Answers: 1

Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2
You know the right answer?
A student was trying to determine the mole percent of A in a mixture of A and B using refractive ind...
Questions in other subjects:


Biology, 19.05.2020 20:10






Mathematics, 19.05.2020 20:10



 = mole fraction of A
= mole fraction of A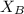 = mole fraction of B
= mole fraction of B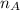 = refractive index of A
= refractive index of A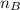 = refractive index of B
= refractive index of B ........ (1)
........ (1)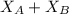 = 1
= 1 ......... (2)
......... (2)