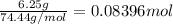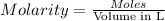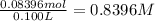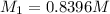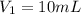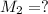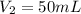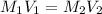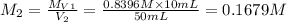Chemistry, 25.02.2020 04:01 swelch2010
. A student transferred a 10-mL aliquot of a stock sodium hypochlorite solution into a 50-mL volumetric flask using a volumetric pipet. The solution was then diluted to the mark with distilled water. Assuming that the concentration of a stock sodium hypochlorite is 6.25% (w/v), calculate the molarity of the diluted sodium hypochlorite solution. Molar mass of sodium hypochlorite

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, maryjane8872
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1


Chemistry, 22.06.2019 17:30, llamasking
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 23.06.2019 00:30, mathwiznot45
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1
You know the right answer?
. A student transferred a 10-mL aliquot of a stock sodium hypochlorite solution into a 50-mL volumet...
Questions in other subjects:










Physics, 14.04.2020 16:09