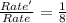Chemistry, 25.02.2020 04:05 alialbinn6969
The gas phase reaction between NO and O2 is second order with respect to NO and first order with respect to 02, what would happen to the reaction rate if the concentrations of both reactants were halved with everything else held constant?
a. It would decrease by a factor of 8 It would remain unchanged.
b. It would decrease by a factor of 16.
c. It would decrease by a factor of 4.
d. It would decrease by a factor of 2

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 17:30, nijanicole164
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 19:30, dorindaramirez0531
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1
You know the right answer?
The gas phase reaction between NO and O2 is second order with respect to NO and first order with res...
Questions in other subjects:


Social Studies, 30.10.2021 07:50


Social Studies, 30.10.2021 07:50

Biology, 30.10.2021 07:50


Mathematics, 30.10.2021 07:50


Mathematics, 30.10.2021 07:50

![Rate=k[NO]^2[O_2]^1](/tpl/images/0522/7517/1df0d.png) (1)
(1)![Rate'=k{\frac{[NO]}{2}^2}{\frac{[O_2]}{2}^1}](/tpl/images/0522/7517/19c10.png) (2)
(2)![\frac{Rate'}{Rate}=\frac{k\frac{[NO]}{2}^2\times \frac{([O_2]}{2})^1}{k[NO]^2[O_2]^1}](/tpl/images/0522/7517/9c613.png)