
Chemistry, 25.02.2020 03:50 vuqepete4528
A particular sample of vinegar has a pH of 2.95. If acetic acid is the only acid that vinegar contains (Ka=1.8×10−5), calculate the concentration of acetic acid in the vinegar.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, miamassimino
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 13:20, alejandra340
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 18:30, chinadoll24
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
A particular sample of vinegar has a pH of 2.95. If acetic acid is the only acid that vinegar contai...
Questions in other subjects:

Mathematics, 27.11.2019 19:31


History, 27.11.2019 19:31



History, 27.11.2019 19:31

English, 27.11.2019 19:31


Mathematics, 27.11.2019 19:31

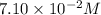
![pH=-\log[H^+]](/tpl/images/0522/6990/cf945.png)
![2.95=-\log[H^+]](/tpl/images/0522/6990/b4bb5.png)
![[H^+]=1.122\times 10^{-3}M](/tpl/images/0522/6990/c196b.png)
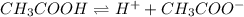
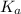 for above equation follows:
for above equation follows:![K_a=\frac{[H^+][CH_3COO^-]}{[CH_3COOH]}](/tpl/images/0522/6990/2a1d5.png)
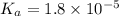
![[H^+]_{eq}=[CH_3COO^-]_{eq}=1.122\times 10^{-3}M](/tpl/images/0522/6990/95208.png)
![1.8\times 10^{-5}=\frac{(1.122\times 10^{-3})\times (1.122\times 10^{-3})}{[CH_3COOH]}](/tpl/images/0522/6990/a71f8.png)
![[CH_3COOH]_{eq}=0.0699M](/tpl/images/0522/6990/8c7a5.png)
![[CH_3COOH]_{eq}+[H^+]_{eq}=(0.0699+0.001122)=7.10\times 10^{-2}M](/tpl/images/0522/6990/444ef.png)



