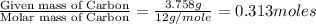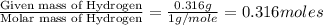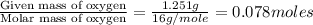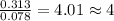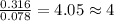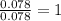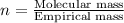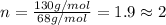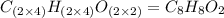Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, stellaglenn205
What reaction is taking place? 02 + c3h8 = h20 + co2
Answers: 1

Chemistry, 22.06.2019 06:30, rosieposie27
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

You know the right answer?
A 5.325g sample of methyl benzoate, a compound in perfumes , was found to contain 3.758 g of carbon,...
Questions in other subjects:


Chemistry, 21.07.2019 16:30


Chemistry, 21.07.2019 16:30

Mathematics, 21.07.2019 16:30



Physics, 21.07.2019 16:30


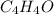 and
and 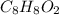 respectively
respectively