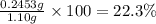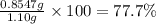Chemistry, 25.02.2020 03:23 alizeleach0123
>A 1.10-g sample contains only glucose (C6H12O6) and sucrose (C12H22O11). When a sample is dissolved in water to a total solution volume of 25.0 mL, the osmotic pressure of the solution is 3.78 atm at 298 K. What is the percent mass of the glucose in the sample, and what is the percent mass of sucrose in the sample?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:30, camerondillonn
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1


Chemistry, 23.06.2019 02:30, erikacastro259
what is your question? collegechemistry 5+3 pts in november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3
You know the right answer?
>A 1.10-g sample contains only glucose (C6H12O6) and sucrose (C12H22O11). When a sample is dissol...
Questions in other subjects:

Mathematics, 08.03.2021 16:10

Mathematics, 08.03.2021 16:10

Mathematics, 08.03.2021 16:10



Mathematics, 08.03.2021 16:10

History, 08.03.2021 16:10

Geography, 08.03.2021 16:10

Mathematics, 08.03.2021 16:10

Computers and Technology, 08.03.2021 16:10

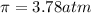
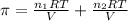
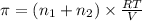
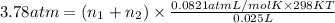
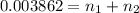
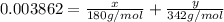
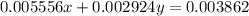 ..[2]
..[2]