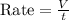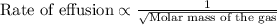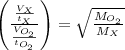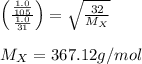A gas of unknown molecular mass was allowed to effuse through a small opening under constant-pressure conditions. It required 105 s for 1.0 L of the gas to effuse. Under identical experimental conditions it required 31 s for 1.0 L of O2 gas to effuse. Calculate the molar mass of the unknown gas.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, themajesty9898
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 14:10, roserose3098
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 15:30, sanchez7489
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1
You know the right answer?
A gas of unknown molecular mass was allowed to effuse through a small opening under constant-pressur...
Questions in other subjects:





Mathematics, 09.06.2021 18:20


Mathematics, 09.06.2021 18:20

Mathematics, 09.06.2021 18:20


Mathematics, 09.06.2021 18:20