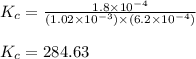Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, breannaasmith1122
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 04:20, lindseysmith9522
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1


You know the right answer?
Consider the following reaction: Fe3+(aq)+SCN−(aq)⇌FeSCN2+(aq) A solution is made containing an init...
Questions in other subjects:

Chemistry, 29.10.2019 05:31

Geography, 29.10.2019 05:31


Biology, 29.10.2019 05:31


Physics, 29.10.2019 05:31

Mathematics, 29.10.2019 05:31



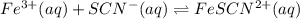 A solution is made containing an initial
A solution is made containing an initial ![[Fe^{3+}]](/tpl/images/0522/5263/5ed68.png) of 1.2×10⁻³ M and an initial [SCN⁻] of 8.0×10⁻⁴ M . At equilibrium, [FeSCN²⁺]= 1.8×10⁻⁴ M.
of 1.2×10⁻³ M and an initial [SCN⁻] of 8.0×10⁻⁴ M . At equilibrium, [FeSCN²⁺]= 1.8×10⁻⁴ M.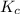 for above equation is 284.63
for above equation is 284.63![[Fe^{3+}]=1.2\times 10^{-3}M](/tpl/images/0522/5263/e152e.png)
![[SCN^{-}]=8.0\times 10^{-4}M](/tpl/images/0522/5263/5f5c1.png)
![[FeSCN^{2+}]=1.8\times 10^{-4}M](/tpl/images/0522/5263/2496b.png)
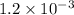
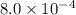
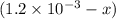
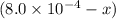 x
x![[Fe^{3+}]=(1.2\times 10^{-3})-(1.8\times 10^{-4)=1.02\times 10^{-3}M](/tpl/images/0522/5263/a018d.png)
![[SCN^{-}]=(8.0\times 10^{-3})-(1.8\times 10^{-4)=6.2\times 10^{-4}M](/tpl/images/0522/5263/febab.png)
![K_c=\frac{[FeSCN^{2+}]}{[Fe^{3+}][SCN^-]}](/tpl/images/0522/5263/417f5.png)