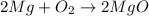Chemistry, 25.02.2020 03:04 tonimgreen17p6vqjq
A student burned 0.250 g of magnesium metal in an open crucible. Oxygen from the air combined with the metal forming magnesium oxide. If 0.421 g of magnesium oxide were produced, what mass of oxygen combined with the metal? 1. 0.171 g 2. 0.671 g 3. 1.684 g 4. 0.594 g

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, perezanthony2403
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 16:00, jrocklove7825
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 23.06.2019 01:00, jaidencoolman2510
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2
You know the right answer?
A student burned 0.250 g of magnesium metal in an open crucible. Oxygen from the air combined with t...
Questions in other subjects:


Mathematics, 07.07.2019 03:00

Spanish, 07.07.2019 03:00

Mathematics, 07.07.2019 03:00

Social Studies, 07.07.2019 03:00


Mathematics, 07.07.2019 03:00


Mathematics, 07.07.2019 03:00

History, 07.07.2019 03:00