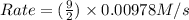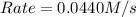The reaction A + B ⟶ C + D rate = k [ A ] [ B ] 2 has an initial rate of 0.0440 M / s. What will the initial rate be if [ A ] is halved and [ B ] is tripled? initial rate: .00978 M / s What will the initial rate be if [ A ] is tripled and [ B ] is halved? initial rate:

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Bradgarner772
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 22.06.2019 14:30, neidaq12345
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2


Chemistry, 22.06.2019 16:00, ghadeeraljelawy
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2
You know the right answer?
The reaction A + B ⟶ C + D rate = k [ A ] [ B ] 2 has an initial rate of 0.0440 M / s. What will the...
Questions in other subjects:

Physics, 08.05.2021 07:30

Mathematics, 08.05.2021 07:30


Mathematics, 08.05.2021 07:30





Mathematics, 08.05.2021 07:30

![Rate=k[A][B]^2](/tpl/images/0522/4296/d8f90.png)
![Rate=k\times (\frac{[A]}{2})\times (3\times [B])^2](/tpl/images/0522/4296/31c7e.png)
![Rate=k\times (\frac{[A]}{2})\times 9\times [B]^2](/tpl/images/0522/4296/e768d.png)
![Rate=k\times (\frac{9}{2})\times [A]\times [B]^2](/tpl/images/0522/4296/4e890.png)
![k[A][B]^2](/tpl/images/0522/4296/8ef61.png) = 0.0440 M/s
= 0.0440 M/s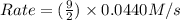
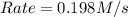
![Rate=k\times (\frac{[B]}{2})\times (3\times [A])^2](/tpl/images/0522/4296/e26df.png)
![Rate=k\times (\frac{[B]}{2})\times 9\times [A]^2](/tpl/images/0522/4296/e83c0.png)