
Chemistry, 25.02.2020 00:50 SucMaDongShan
For a particular reaction at 298 K, the equilibrium constant is equal to 581. Determine ΔG° in kJ/mol for the reaction. Do not include units. Report your answer to 3 significant figures.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 15:00, makaylajones74pdxtrk
What is the most important factor in determining climates.
Answers: 1

Chemistry, 23.06.2019 01:00, tjeffers90028
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3
You know the right answer?
For a particular reaction at 298 K, the equilibrium constant is equal to 581. Determine ΔG° in kJ/mo...
Questions in other subjects:




Mathematics, 11.01.2021 23:00




History, 11.01.2021 23:00

Mathematics, 11.01.2021 23:00

Biology, 11.01.2021 23:00


 = Standard Gibbs free energy = ?
= Standard Gibbs free energy = ?
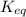 = equilibrium constant = 581
= equilibrium constant = 581


