Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:40, loveoneonly9153
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 14:40, elawnnalewis4855
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 19:50, jakaylathomas11
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 23.06.2019 01:30, AptAlbatross
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3
You know the right answer?
Nitroglycerine decomposes violently according to the chemical equation below. What mass of carbon di...
Questions in other subjects:

Mathematics, 03.02.2020 05:59



Business, 03.02.2020 05:59


English, 03.02.2020 05:59

Mathematics, 03.02.2020 05:59


Social Studies, 03.02.2020 05:59

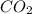 produced is, 7.74 grams
produced is, 7.74 grams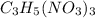 = 13.3 g
= 13.3 g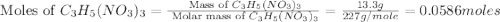
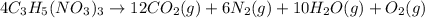
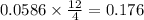 moles of
moles of