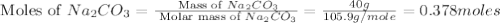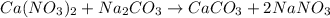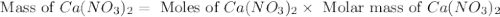Chemistry, 25.02.2020 00:41 melissareid65
Redo the experiment by clicking on Reset Experiment. Add Ca(NO3)2 to 40 g of Na2CO3 and determine at what point the masses of the two reactants react "evenly." That is, how many grams of Ca(NO3)2 must be added to just consume the 40 g Na2CO3 initially available?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, chloe081
We just started a new lesson in chemistry and everyone hates it and i dont get it one bit. i hate school. h el p. balanced equationc3h8+5o2-> 3co2+4h2o1.) if you start with 14.8g of propane(c3h8) and 3.44g of oxygen, which is the limiting reactant -check my answer 2.)what mass of excess reagent is left over? 3.)what mass of carbon dioxide can be made? 4.)what mass of water is produced?
Answers: 2


Chemistry, 22.06.2019 13:30, princessroseee769
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

You know the right answer?
Redo the experiment by clicking on Reset Experiment. Add Ca(NO3)2 to 40 g of Na2CO3 and determine at...
Questions in other subjects:


History, 04.07.2019 18:00






Mathematics, 04.07.2019 18:00


Mathematics, 04.07.2019 18:00

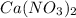 added must be, 61.9 grams
added must be, 61.9 grams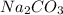 = 40 g
= 40 g