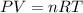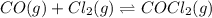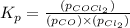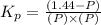Chemistry, 24.02.2020 22:28 pattydixon6
Pure phosgene gas (COCl2,), 3.30 x 10-2 mol, was placed in a 1.50-L container. It was heated to 800K, and at equilibrium the pressure of CO was found to be 0.497 atm. Calculate the equilibrium constant, Kp, for the reaction: CO(g) Cl2(g) COCl2(g)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, flakko1899
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1


Chemistry, 22.06.2019 19:10, aamu15
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3

Chemistry, 23.06.2019 14:30, hhhhhh8897
If 125 cal of heat is applied to a 60.0-g piece of copper at 25.0 ? c , what will the final temperature be? the specific heat of copper is 0.0920 cal/(g? ? c) .
Answers: 1
You know the right answer?
Pure phosgene gas (COCl2,), 3.30 x 10-2 mol, was placed in a 1.50-L container. It was heated to 800K...
Questions in other subjects:


Geography, 19.08.2019 20:30

Social Studies, 19.08.2019 20:30




Chemistry, 19.08.2019 20:30



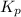 for the reaction is, 3.82
for the reaction is, 3.82