
Chemistry, 24.02.2020 19:29 hannahkharel2
The rate constant, k, for a first-order reaction is equal to 4.2 × 10-4 s-1. What is the half-life for the reaction?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:30, lizzyhearts
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 23.06.2019 00:00, queenpaige2015
Which samples do the atoms have the least kinetic energy
Answers: 2

Chemistry, 23.06.2019 03:10, mani1682
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2
You know the right answer?
The rate constant, k, for a first-order reaction is equal to 4.2 × 10-4 s-1. What is the half-life f...
Questions in other subjects:






Biology, 25.02.2021 20:50

History, 25.02.2021 20:50

English, 25.02.2021 20:50

Mathematics, 25.02.2021 20:50

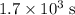 .
. 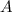 .) The half-life
.) The half-life 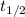 of that first-order reaction would be the time it takes for
of that first-order reaction would be the time it takes for ![[A]](/tpl/images/0521/5010/6aa06.png) (concentration of
(concentration of 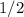 its initial value.
its initial value.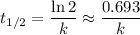 ,
, 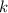 is the rate constant of this reaction.
is the rate constant of this reaction.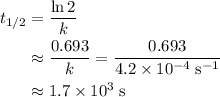 .
.

