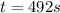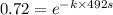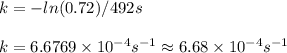Chemistry, 24.02.2020 18:41 kprincess16r
The isomerization reaction CH3NC → CH3CN obeys the first-order rate law, rate = k[CH3NC], in the presence of an excess of argon. Measurement at 500. K reveals that in 492 seconds, the concentration of CH3NC has decreased to 72% of its original value. Calculate the rate constant (k) of the reaction at 500. K.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, KnMcdonaldk93906
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 22.06.2019 10:10, ragegamer334p3xlso
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3


Chemistry, 22.06.2019 13:00, torigirl4126
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3
You know the right answer?
The isomerization reaction CH3NC → CH3CN obeys the first-order rate law, rate = k[CH3NC], in the pre...
Questions in other subjects:

Chemistry, 17.07.2019 10:50



Mathematics, 17.07.2019 10:50

English, 17.07.2019 10:50


Health, 17.07.2019 10:50



History, 17.07.2019 10:50

![\dfrac{d[CH_3NC]}{[CH_3NC]}=-kt](/tpl/images/0521/4081/74427.png)
![[CH_3NC]=[CH_3NC]_0e^{-kt}](/tpl/images/0521/4081/d933f.png)
![\dfrac{CH_3NC]}{[CH_3NC]_0}=0.72](/tpl/images/0521/4081/eda95.png)