Chemistry, 24.02.2020 18:28 ninja12302
Lithium nitride reacts with water to produce ammonia and lithium hydroxide according to the equation Li 3 N ( s ) + 3 H 2 O ( l ) ⟶ NH 3 ( g ) + 3 LiOH ( aq ) Heavy water is water with the isotope deuterium in place of ordinary hydrogen, and its formula is D 2 O . The same reaction can be used to produce heavy ammonia, ND 3 ( g ) , according to the equation Li 3 N ( s ) + 3 D 2 O ( l ) ⟶ ND 3 ( g ) + 3 LiOD ( aq ) Calculate how many grams of heavy water are required to produce 320.0 mg ND 3 ( g ) . The mass of deuterium, D , is 2.014 g/mol.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:50, limelight11
Which statement describes how phase changes can be diagrammed as a substance is heated? the phase is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the phase is on the x-axis. the time is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the time is on the x-axis.
Answers: 1


Chemistry, 22.06.2019 12:30, poopybutt541
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 13:30, kassandrarosario1115
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1
You know the right answer?
Lithium nitride reacts with water to produce ammonia and lithium hydroxide according to the equation...
Questions in other subjects:

Mathematics, 16.10.2019 02:00


Mathematics, 16.10.2019 02:00


Mathematics, 16.10.2019 02:00



Mathematics, 16.10.2019 02:00

History, 16.10.2019 02:00

Mathematics, 16.10.2019 02:00

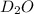 needed is 0.96 grams
needed is 0.96 grams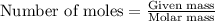 .....(1)
.....(1)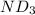 :
: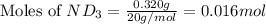
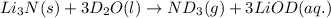
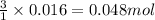 of
of