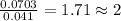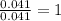Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, sdlesley66
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

You know the right answer?
A compound is composed of C, H and O. A 1.621 g sample of this compound was combusted, producing 1.9...
Questions in other subjects:



Mathematics, 10.12.2021 23:10


Mathematics, 10.12.2021 23:10



Mathematics, 10.12.2021 23:10

English, 10.12.2021 23:10





 of carbon dioxide,
of carbon dioxide, 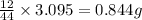 of carbon will be contained.
of carbon will be contained. of water,
of water, 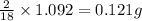 of hydrogen will be contained.
of hydrogen will be contained.![(1.621)-[(0.844)+(0.121)]=0.656g](/tpl/images/0521/3218/1f9d2.png)

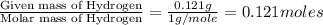

 moles.
moles.