
The fuel used to power the booster rockets on space shuttles is a mixture of aluminum metal and ammonium perchlorate. the following balanced equation represents the reaction.. 3al + 3nh4clo4 → al2o3 + alcl3 + 3no + 6h2o how many moles of water are produced from 373 mol al?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:00, jazmine8194
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1


Chemistry, 23.06.2019 04:31, saladdressing1425
Chemical engineering who specializes in negotiating for large purchases and instructing customers in use of the products are
Answers: 1
You know the right answer?
The fuel used to power the booster rockets on space shuttles is a mixture of aluminum metal and ammo...
Questions in other subjects:


Mathematics, 04.06.2020 21:01





Mathematics, 04.06.2020 21:01



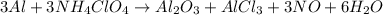
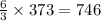 moles of water
moles of water

