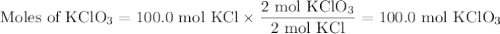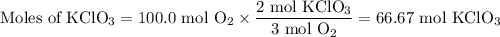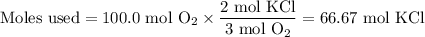Chemistry, 24.02.2020 07:21 DASASDAEDWEDA
Consider the following chemical reaction: 2KCl + 3O2 --> 2KClO3. If you are given 100.0 moles of KCl and 100.0 moles of O2...
what is the limiting reactant? (TYPE EITHER KCl or O2)
what is the excess reactant? (TYPE EITHER KCl or O2)
how many moles of the excess reactant will be left over? moles (TYPE just the number to the correct amount of significant figures)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, aleilyg2005
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3



Chemistry, 23.06.2019 05:00, MoneyMike42
Select the statement that describe chemical properties a. antacid tablets neutralize stomach acid b. helium is the lightest monatomic element c. water freezes at 0 celsius d. mercury is liquid at room temperature
Answers: 3
You know the right answer?
Consider the following chemical reaction: 2KCl + 3O2 --> 2KClO3. If you are given 100.0 moles of...
Questions in other subjects:


History, 10.02.2021 05:20


Mathematics, 10.02.2021 05:20

Physics, 10.02.2021 05:20

Chemistry, 10.02.2021 05:20

Mathematics, 10.02.2021 05:20

Mathematics, 10.02.2021 05:20


Mathematics, 10.02.2021 05:20