
Chemistry, 24.02.2020 06:45 kdenormandie3122
If 3.87g of powdered aluminum oxide is placed in a container containing 5.67g of water, what is the limiting reactant?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, alexandroperez13
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 01:10, mistiehaas
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2


You know the right answer?
If 3.87g of powdered aluminum oxide is placed in a container containing 5.67g of water, what is the...
Questions in other subjects:

Mathematics, 11.06.2020 21:57

Mathematics, 11.06.2020 21:57


Mathematics, 11.06.2020 21:57






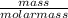
 = 0.04mole
= 0.04mole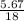 = 0.32mole
= 0.32mole

