
Chemistry, 24.02.2020 05:09 sheltongraham1011
A sample of krypton gas occupies 18.60 l at a pressure of 5.540 atm and 35.00 c. what volume would the krypton occupy at stop

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, SchoolFirst9811
The scheme below is from a series of reactions that are part of a synthesis of vitamin a. answer the following questions with reference to this scheme. (i) what is "reagent a"? (ii) draw a step-by-step mechanism which explains the formation of compound c from compound b (iii) which reagents would you use to form compound e from compounds c and d (reagents b and c)? for each reagent suggested above in (ii) explain the role of the reagent in the reaction to (iv) form compound e. you may wish to do this by drawing a mechanism. 1. addition of reagent a но reagent a 2. н, о" thо oh нон-с compound a. compound b. compound c .ch-оh 1. reagent b "сно 2. reagent c сh oh compound e. compound d.
Answers: 2

Chemistry, 23.06.2019 06:00, tytianadyson74
What volume of argon gas is equal to 1.60 grams of argon
Answers: 1

Chemistry, 23.06.2019 06:00, wirchakethan23
When hydrogen peroxide (h2o2) is added to potassium iodide (ki) solution, the hydrogen peroxide decomposes into water (h2o) and oxygen (o2). the chemical equation for the decomposition reaction is: 2h2o2—> 2h2o + o2. what is the role of the potassium iodide in this reaction? a. reactant. b. product. c. precipitate. d. catalyst.
Answers: 1
You know the right answer?
A sample of krypton gas occupies 18.60 l at a pressure of 5.540 atm and 35.00 c. what volume would t...
Questions in other subjects:


Health, 26.07.2021 09:10

English, 26.07.2021 09:10

Social Studies, 26.07.2021 09:10


English, 26.07.2021 09:10


Social Studies, 26.07.2021 09:10


History, 26.07.2021 09:10

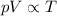

 (initial pressure)
(initial pressure) (initial volume)
(initial volume)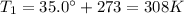 (initial temperature)
(initial temperature) (final pressure, at stp)
(final pressure, at stp) (temperature at stp)
(temperature at stp)


