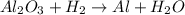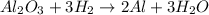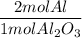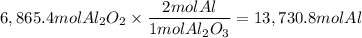Answers: 1


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 04:00, Ezekielcassese
Which method would be best to separate a mixture of sand and gravel
Answers: 1

Chemistry, 23.06.2019 04:31, 24swimdylanoh
What are the coefficients that will balance the skeleton equation below? n2 + h2 → nh3
Answers: 1

Chemistry, 23.06.2019 07:20, aprilkenedy12
Which of the following are acids or bases? 1. sodium hydrogen 2. barium hydroxide solution 3. carbonate solution
Answers: 1
You know the right answer?
A process to produce aluminum from aluminum oxide has an 85.0% yield. How much aluminum will be prod...
Questions in other subjects:



Mathematics, 13.01.2020 01:31

Mathematics, 13.01.2020 01:31

Chemistry, 13.01.2020 01:31


Mathematics, 13.01.2020 01:31

Mathematics, 13.01.2020 01:31

Mathematics, 13.01.2020 01:31

Mathematics, 13.01.2020 01:31