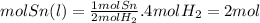At 1023 K and 1 atm, a 3.00 gram sample of Snoz(s) (gram-formula mass = 151 g/mol) reacts with hydrogen gas to produce tin and water, as
shown in the balanced equation below:
SnO2 (s) + 2H2(g) + Sn(l) + 2H2O(g)
Determine the number
of moles of sn(l)
produced when 4.0
Moles of H2(g) is completely consumed

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 05:00, hjamya17
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

You know the right answer?
At 1023 K and 1 atm, a 3.00 gram sample of Snoz(s) (gram-formula mass = 151 g/mol) reacts with hydro...
Questions in other subjects:


History, 05.10.2020 15:01

Mathematics, 05.10.2020 15:01


English, 05.10.2020 15:01



Arts, 05.10.2020 15:01

English, 05.10.2020 15:01

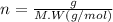
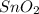 (s) + 2
(s) + 2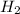 (g) ⇒ Sn(l) + 2
(g) ⇒ Sn(l) + 2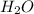 (g)
(g)