WILL MARK BRAINLIEST FOR CORRECT ANSWER!
Methane burns in oxygen to produce carbon diox...

WILL MARK BRAINLIEST FOR CORRECT ANSWER!
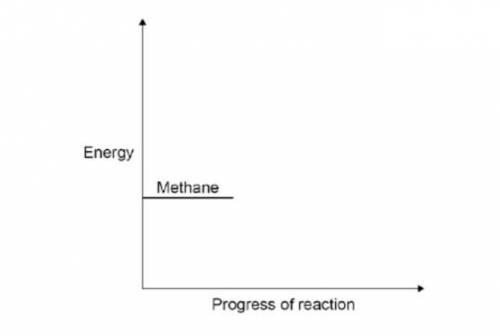
Methane burns in oxygen to produce carbon dioxide and water. The activation energy for the reaction is 2648 kJ / mol. The reaction give 818 kJ / mol. The reaction gives out 818 kJ / mol of energy. Complete the reaction profile. Draw arrows to represent:
the activation energy
the energy given out.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, peternice2956
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 19:00, ecolifesfsu1263
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1


Chemistry, 23.06.2019 07:00, bree6754
Achemist who studies water samples did a demonstration of how to test for lead in water. she added a clear solution of potassium iodide to a clear solution of lead nitrate. then a yellow swirling solid formed in the liquid. what is most likely true about the yellow solid?
Answers: 3
You know the right answer?
Questions in other subjects:

Mathematics, 14.01.2020 17:31





Mathematics, 14.01.2020 17:31

Health, 14.01.2020 17:31

English, 14.01.2020 17:31

Health, 14.01.2020 17:31

Mathematics, 14.01.2020 17:31



