

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:00, sandeebassett3
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2


Chemistry, 23.06.2019 16:30, Person51761
Which model represents the process of nuclear fusion?
Answers: 1
You know the right answer?
How many milliliters of 2.36 M copper nitrate would be needed to react with 250 mL of 0.519 M silver...
Questions in other subjects:



Biology, 15.12.2019 18:31




Health, 15.12.2019 18:31


History, 15.12.2019 18:31

History, 15.12.2019 18:31

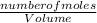
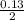 = 0.07mole of Cu(NO₃)₂
= 0.07mole of Cu(NO₃)₂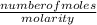 =
= 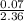 = 0.028dm³
= 0.028dm³

