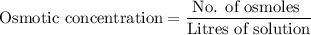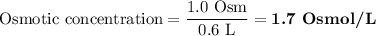Chemistry, 23.02.2020 04:42 PrincessIndia
A solution contains 0.5 moles of MgSO4 dissolved in 0.6 liters of water. In water MgSO4 dissociates into one Mg2 particle and one SO42- particle. What is the osmolarity of the solution?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, lucasrandall
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 09:30, mazielynn84
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 10:10, babyphoraaaaa
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate, m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

You know the right answer?
A solution contains 0.5 moles of MgSO4 dissolved in 0.6 liters of water. In water MgSO4 dissociates...
Questions in other subjects:


Mathematics, 11.01.2021 19:00

Mathematics, 11.01.2021 19:00


History, 11.01.2021 19:00

English, 11.01.2021 19:00



Mathematics, 11.01.2021 19:00