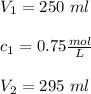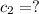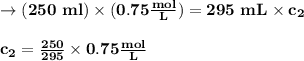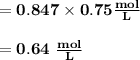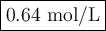Chemistry, 23.02.2020 04:47 brainlord4209
Dilutions Worksheet - Solutions
If 45 mL of water are added to 250 mL of a 0.75 M K2SO4 solution, what will the
molarity of the diluted solution be?
(0.75 M)(250 ml) = M2 (295 mL)
M2 = (0.75 M) (250 mL) = 0.64 M
(295 mL)
Where did the 295ml came from

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, andybiersack154
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 00:00, lilyclairehutson
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

You know the right answer?
Dilutions Worksheet - Solutions
If 45 mL of water are added to 250 mL of a 0.75 M K2SO4 soluti...
If 45 mL of water are added to 250 mL of a 0.75 M K2SO4 soluti...
Questions in other subjects:

Health, 07.07.2019 09:30

Mathematics, 07.07.2019 09:30


Mathematics, 07.07.2019 09:30

Mathematics, 07.07.2019 09:30

English, 07.07.2019 09:30

English, 07.07.2019 09:30

English, 07.07.2019 09:30

Physics, 07.07.2019 09:30

Mathematics, 07.07.2019 09:30