Chemistry, 23.02.2020 00:22 JamierW2005
Using the symbol below/above determine the number of protons, neutrons, and electrons in the element.
A. Protons = 50
Neutrons = 118
Protons = 46
B. Protons = 68
Neutrons = 46
Electrons = 54
C. Protons = 50
Neutrons = 68
Electrons = 46
D. Protons = 118
Neutrons = 50
Electrons = 46
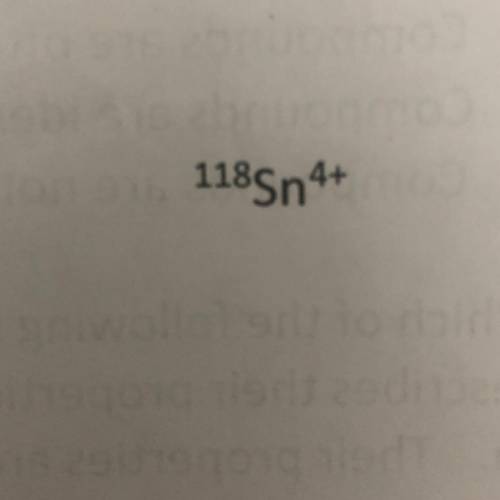

Answers: 1


Other questions on the subject: Chemistry



You know the right answer?
Using the symbol below/above determine the number of protons, neutrons, and electrons in the element...
Questions in other subjects:

Mathematics, 07.01.2020 15:31

Chemistry, 07.01.2020 15:31

Mathematics, 07.01.2020 15:31

History, 07.01.2020 15:31



Mathematics, 07.01.2020 15:31

Mathematics, 07.01.2020 15:31

Biology, 07.01.2020 15:31