Chemistry, 22.02.2020 04:36 sarahgarza5440
What is the relative atomic mass of a hypothetical element that consists of the following isotopes in the indicated natural abundances? Isotope - Isotopic mass (amuamu) - Relative abundance (%%) 1 - 78.9 - 12.6 2 - 80.9 - 13.9 3 - 82.9 - 73.5

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, zamirareece17
1. list three scientific reasons cockroaches may fly.
Answers: 1



Chemistry, 22.06.2019 23:00, lulprettyb
What is the most common reason for matter changing its state?
Answers: 1
You know the right answer?
What is the relative atomic mass of a hypothetical element that consists of the following isotopes i...
Questions in other subjects:






Mathematics, 28.01.2021 22:30


Geography, 28.01.2021 22:30

Mathematics, 28.01.2021 22:30

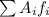
 is the atomic mass of each isotope and
is the atomic mass of each isotope and 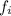 the relative frequency. Therefore, we find:
the relative frequency. Therefore, we find: