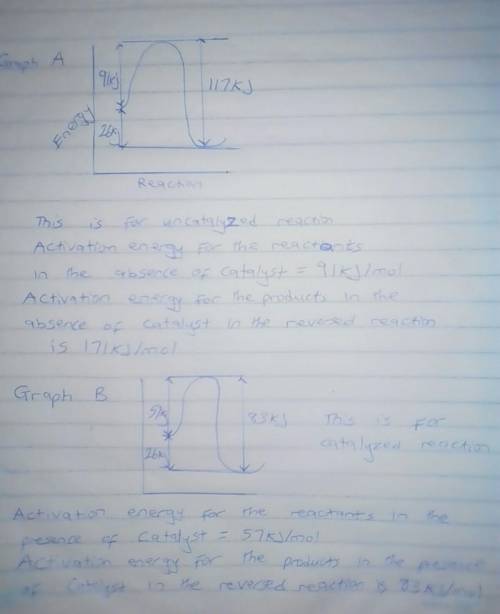
Chemistry, 22.02.2020 02:58 borgesalfonso12
A catalyst decreases the activation energy of a particular exothermic reaction by 34 kJ/mol, to 57 kJ/mol. Assuming that the mechanism has only one step, and that the products are 26 kJ lower in energy than the reactants, sketch approximate energy-level diagrams for the catalyzed and uncatalyzed reactions. What is the activation energy for the uncatalyzed reverse reaction?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, joelpimentel
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 11:40, jerrysandoval22
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 18:30, chinadoll24
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
A catalyst decreases the activation energy of a particular exothermic reaction by 34 kJ/mol, to 57 k...
Questions in other subjects:

Mathematics, 17.05.2021 21:20

Mathematics, 17.05.2021 21:20

Mathematics, 17.05.2021 21:20



Mathematics, 17.05.2021 21:20

Mathematics, 17.05.2021 21:20



Mathematics, 17.05.2021 21:20