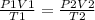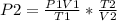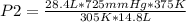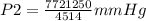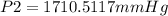Chemistry, 22.02.2020 02:28 jaksmmwlqlzm
A sample of gas with an initial volume of 28.4 Liters at a pressure of 725 mmHg and a temperature of 305 K is compressed to a volume of 14.8 Liters and warmed to a temperature of 375 Kelvin. What is the final pressure of the gas?

Answers: 1


Other questions on the subject: Chemistry


You know the right answer?
A sample of gas with an initial volume of 28.4 Liters at a pressure of 725 mmHg and a temperature of...
Questions in other subjects:


History, 12.11.2020 21:50




Mathematics, 12.11.2020 21:50


Chemistry, 12.11.2020 21:50


Mathematics, 12.11.2020 21:50