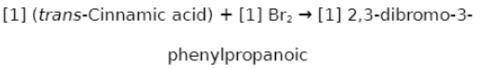Chemistry, 22.02.2020 02:06 jennyferluna0216
Suppose a student started with 142.0 mg of trans-cinnamic acid, 412 mg of pyridinium tribromide, and 2.30 mL of glacial acetic acid. After the reaction and workup, the student ended up with 0.2223 g of brominated product. Calculate the student\'s theoretical and percent yields.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, annafellows
Match term definition ellipse a) diagonal cross section of a cylinder circle b) diagonal cross section through the widest part of a sphere sphere c) cross section parallel to the base of a cone great circle d) shape created when a semi-circle is rotated around the y-axis triangle e) perpendicular cross section of a cone
Answers: 1

Chemistry, 22.06.2019 09:10, chloeholt123
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 20:30, ashley4329
Select all the correct answers. which compounds have the empirical formula ch20? (multiple answers)a. c2h4o2b. c3h603c. ch2o2d. c5h1005e. c6h1206
Answers: 2
You know the right answer?
Suppose a student started with 142.0 mg of trans-cinnamic acid, 412 mg of pyridinium tribromide, and...
Questions in other subjects:


Mathematics, 24.07.2020 01:01