

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, lil3114
Imagine that you own a property that is exactly 2.2 acres large. you want to sell your property, but your realtor tells you that you cannot sell your land by the acre. in order to sell your land you need to determine the area you own in units of square meters? given that there are 1.6 kilometers in 1 mile and 640 acres in 1 square mile, what is the area of land that you own in square meters square meters?
Answers: 2


Chemistry, 22.06.2019 17:00, davisnaziyahovz5sk
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 22.06.2019 20:00, AaronEarlMerringer
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
Order the following compounds according to their expected lattice energies: AlBr3,MgBr2, LiBr, and C...
Questions in other subjects:

History, 30.10.2020 22:40

Mathematics, 30.10.2020 22:40



Mathematics, 30.10.2020 22:40

Mathematics, 30.10.2020 22:40



Mathematics, 30.10.2020 22:40

Mathematics, 30.10.2020 22:40

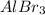 > CaO >
> CaO > 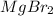 > LiBr
> LiBr

