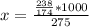Potassium hydrogen phosphate is used in the preparation of non-dairy powdered creamers. Calculate the molarity of a solution prepared by dissolving 238 g of K2HPO4 in enough water to produce 275 mL of solution. A) 0.732 M B) 0.865 M. C) 2.66 M D) 4.97 M E) none of the above

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, clairebear66
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 07:10, jasondesatnick
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 15:30, 20cschultz
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 16:50, briansalazar17
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3
You know the right answer?
Potassium hydrogen phosphate is used in the preparation of non-dairy powdered creamers. Calculate th...
Questions in other subjects:


Mathematics, 06.05.2021 02:20

Mathematics, 06.05.2021 02:20



Biology, 06.05.2021 02:20



Arts, 06.05.2021 02:20

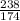 moles.
moles.