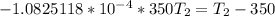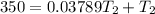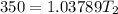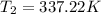Chemistry, 22.02.2020 00:13 jak000067oyyfia
The normal boiling point of liquid ethyl acetate is 350 K. Assuming that its molar heat of vaporization is constant at 34.4 kJ/mol, the boiling point of CH3COOC2H5 when the external pressure is 0.639 atm is K.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:10, dhailyortegacampa131
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 10:30, kluckey3426
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1
You know the right answer?
The normal boiling point of liquid ethyl acetate is 350 K. Assuming that its molar heat of vaporizat...
Questions in other subjects:

English, 01.11.2019 03:31

History, 01.11.2019 03:31

Computers and Technology, 01.11.2019 04:31


Computers and Technology, 01.11.2019 04:31


English, 01.11.2019 04:31

Chemistry, 01.11.2019 04:31


Mathematics, 01.11.2019 04:31

![In\frac{P_2}{P_1}=\frac{\delta H_{vap}}{R}[\frac{T_2-T_1}{T_2T_1}]](/tpl/images/0519/8584/04ef0.png)
![In\frac{0.639}{1}=(\frac{34.4*10^3J/mol}{8.314 J K^{-1}mol^{-1}})[\frac{T_2-350}{350T_2}]](/tpl/images/0519/8584/b0f94.png)
![In({0.639})=(\frac{34.4*10^3}{8.314K^{-1}})[\frac{T_2-350}{350T_2}]](/tpl/images/0519/8584/3464f.png)
![- 0.4479 = 41317.599 [\frac{T_2-350}{350T_2} ]K](/tpl/images/0519/8584/1e76b.png)
![-\frac{0.4479}{4137.599} = [\frac{T_2-350}{350T_2} ]](/tpl/images/0519/8584/1ae08.png)
![- 1.0825118*10^{-4} = [\frac{T_2-350}{350T_2} ]](/tpl/images/0519/8584/1627c.png)