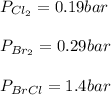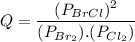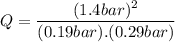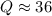Chemistry, 21.02.2020 23:49 Katlynnmarkle13
Consider the gas-phase reaction, Cl2(g) + Br2(g) <=> 2 BrCl(g), for which Kp = 32 at 500 K. If the mixture is analyzed and found to contain 0.19 bar of Cl2, 0.29 bar of Br2 and 1.4 bar of BrCl, describe the situation:a) Q > K and more reactants will be made to reach equilibrium. b) Q > K and more products will be made to reach equilibrium. c) Within 1 decimal place, Q = K and the reaction is at equilibriumd) Q < K and more products will be made to reach equilibrium. e) Q < K and more reactants will be made to reach equilibrium.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 13:00, nadiarose6345
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 19:50, nikoidurrant
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2
You know the right answer?
Consider the gas-phase reaction, Cl2(g) + Br2(g) <=> 2 BrCl(g), for which Kp = 32 at 500 K. If...
Questions in other subjects:






History, 25.11.2019 08:31

Biology, 25.11.2019 08:31

Social Studies, 25.11.2019 08:31

Mathematics, 25.11.2019 08:31

Mathematics, 25.11.2019 08:31