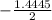Chemistry, 21.02.2020 23:50 westlakebuddy1229
Picric acid has been used in the leather industry and in etching copper. However, its laboratory use has been restricted because it dehydrates on standing and can become shock sensitive. It has an acid dissociation constant of 0.42.
a. What is the [H3O+] for an 0.52 M solution of picric acid? Enter to 4 decimal places.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, officialrogerfp3gf2s
How to solve 4 nh3(g) + 5 o2(g) > 4 no(g) + 6 h2o(g) in chemistry
Answers: 1

Chemistry, 22.06.2019 20:00, bbyjean9974
State one important difference between a physical change and a chemical change?
Answers: 1

Chemistry, 23.06.2019 03:00, jaidencoolman2866
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1

Chemistry, 23.06.2019 03:30, alvfran1041
Astudent uses universal ph paper to find the ph of three solutions . solution a has a ph of 5 solution b has a ph of 11 and solution c has a ph of 7 identify which solution is acidic which solution is neutral and which solution is basic
Answers: 1
You know the right answer?
Picric acid has been used in the leather industry and in etching copper. However, its laboratory use...
Questions in other subjects:





English, 21.05.2021 06:00

Mathematics, 21.05.2021 06:00




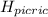
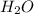 ⇄
⇄ 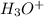 +
+ 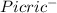
 ) = 0.42
) = 0.42![K_a = \frac{[H_3O^+][Picric^-]}{H_{picric}}](/tpl/images/0519/8296/7a480.png)
![0.42 = \frac{[x][x]}{0.52-x}}](/tpl/images/0519/8296/03f90.png)
![0.42 = \frac{[x]^2}{0.52-x}}](/tpl/images/0519/8296/6158a.png)
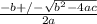 ; ( where +/- represent ± )
; ( where +/- represent ± )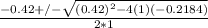
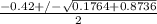
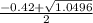 OR
OR 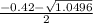
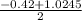 OR
OR 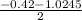
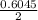 OR
OR