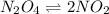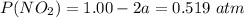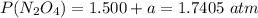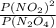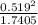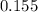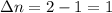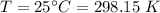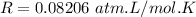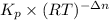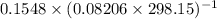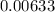Chemistry, 21.02.2020 22:19 hartzpeyton136
A flask is charged with 1.500atm of N2O4(g) and 1.00 atm NO2(g) at 25 degree C, and the following equilibrium is achieved: N2O4(g) 2NO2 After equilibrium is reached, the partial pressure of NO2 is 0.519atm. Calculate the value of Kp for the reaction. Calculate Kc for the reaction.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:40, shanicar33500
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 12:10, kaitlynbernatz2778
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 22:10, steven0448
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1

Chemistry, 23.06.2019 04:00, zakarycrane8101
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 3
You know the right answer?
A flask is charged with 1.500atm of N2O4(g) and 1.00 atm NO2(g) at 25 degree C, and the following eq...
Questions in other subjects:

Mathematics, 14.07.2020 07:01

English, 14.07.2020 07:01


Mathematics, 14.07.2020 07:01

Mathematics, 14.07.2020 07:01


Mathematics, 14.07.2020 07:01

Mathematics, 14.07.2020 07:01


Mathematics, 14.07.2020 07:01