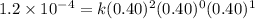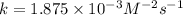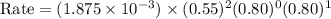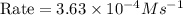Chemistry, 21.02.2020 19:53 faithtunison
For the reaction A+B+C→D+E, the initial reaction rate was measured for various initial concentrations of reactants. The following data were collected:
Trial [A]
(M) [B]
(M) [C]
(M) Initial rate
(M/s)
1 0.40 0.40 0.40 1.2×10−4
2 0.40 0.40 1.20 3.6×10−4
3 0.80 0.40 0.40 4.8×10−4
4 0.80 0.80 0.40 4.8×10−4
Given the data calculated in Parts A, B, C, and D, determine the initial rate for a reaction that starts with 0.55 M of reagent A and 0.80 M of reagents B and C?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, catdog5225
Drive down any three characteristic of modern periodic table
Answers: 1


Chemistry, 22.06.2019 12:00, daytonalive83481
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 17:30, kevin72937
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1
You know the right answer?
For the reaction A+B+C→D+E, the initial reaction rate was measured for various initial concentration...
Questions in other subjects:

Biology, 14.01.2021 14:00




History, 14.01.2021 14:00

Mathematics, 14.01.2021 14:00

Spanish, 14.01.2021 14:00

Mathematics, 14.01.2021 14:00


Mathematics, 14.01.2021 14:00

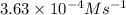
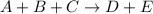
![\text{Rate}=k[A]^a[B]^b[C]^c](/tpl/images/0519/4605/be89a.png)
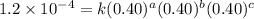 ....(1)
....(1)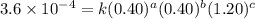 ....(2)
....(2)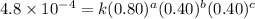 ....(3)
....(3)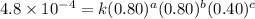 ....(4)
....(4)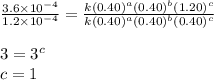
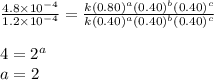
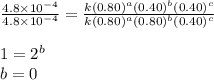
![\text{Rate}=k[A]^2[B]^0[C]^1](/tpl/images/0519/4605/54afd.png)